
+90 212 257 03 21-26
Kultur Mahallesi, Kucuk Camlik Sitesi Esra Sokak No: 2A, A1 Blok, D:10 K:4, 34337 Etiler, Istanbul – TURKEY
 Our Mission
Our Mission
To advance clinical research by delivering high‑quality, compliant, and efficient solutions that support sponsors throughout the clinical trial lifecycle, while prioritizing patient safety and data integrity.
 Our Commitment
Our Commitment
We are committed to scientific integrity, regulatory excellence, and transparent collaboration—ensuring reliable results, patient‑centered conduct, and sustainable partnerships

 Regional Clinical Research Coverage
Regional Clinical Research Coverage
EBM provides high-quality, compliant clinical research services to global and local sponsors across Türkiye, the Middle East, and West Asia, supporting pharmaceutical, biotechnology, and medical device development with strong regional expertise
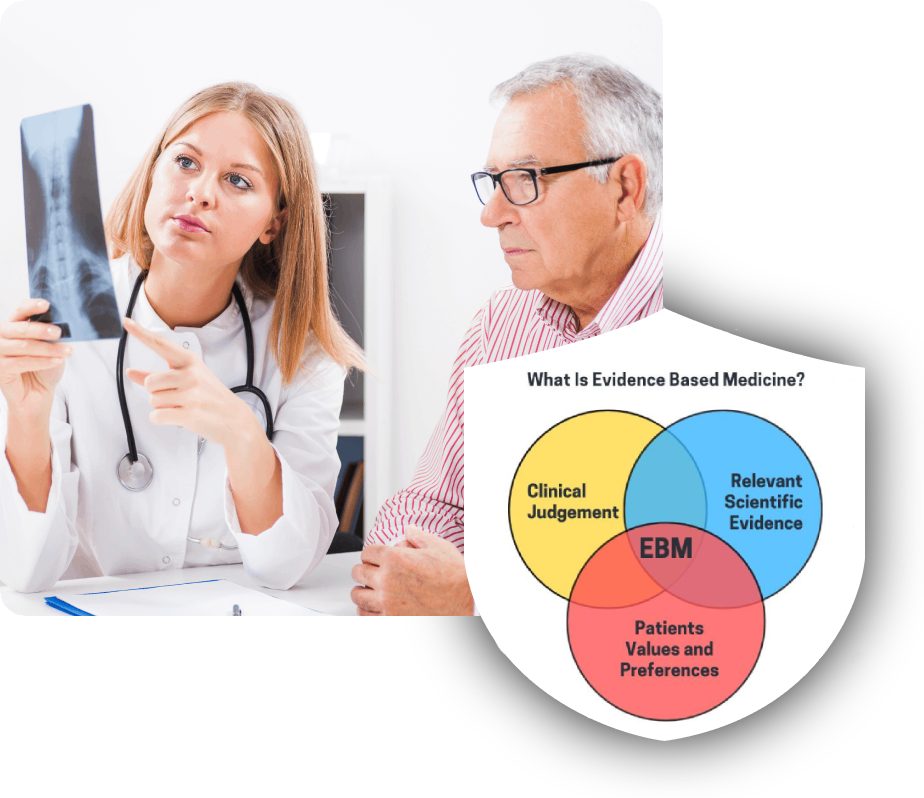

We are specialized in conducting Phase II to Phase IV clinical studies with our highly skilled clinical research team, providing global experience and local expertise.
who had gained their experience as employees of International CROs and Pharmaceutical Companies. Our main objective is to establish a strong bridge between international clients and local expert Clinical Research staff who has Global Experience and Local Expertise.



This recognition is given to hospitals that have achieved high ratings for clinical quality and patient safety across multiple specialties and procedures.
This recognition is given to hospitals that have met rigorous standards for patient safety and quality of care.
All services are conducted under a fully controlled Standard Operating Procedures (SOP) framework aligned with current ICH-GCP, national regulations, and global quality standards, ensuring consistency, regulatory compliance, and continuous audit readiness.
EBM provides comprehensive clinical research services to pharmaceutical companies, supporting regulatory compliance, operational excellence, and the generation of reliable, high-quality data across clinical study phases.
Certified translation vendors are engaged to provide accurate, consistent, and regulation-compliant translations of clinical and regulatory documents, in line with applicable local and international requirements.
EBM works with authorized pharmacy vendors, in accordance with national regulations, to support, where applicable, the controlled procurement, distribution, and tracking of concomitant medications and/or standard-of-care products.
EBM collaborates with Turkish Medicines and Medical Devices Agency–approved licensed depots to ensure compliant storage, handling, and distribution of investigational products in accordance with applicable regulatory and quality standards.
EBM is a Clinical Research Services provider for Pharmaceutical, Biotechnology and Medical Device companies and International Research Organizations. We specialize in conducting Phase Il to Phase IV clinical studies with our highly skilled clinical research team, providing global experience and local expertise. Our logo represents the importance of Evidence Based Medicine in the Medical Sciences, especially in Clinical Research. EBM Clinical Research Services Ltd. has been established by Experienced Clinical Research Team who had gained their experience as employees of International CROs and Pharmaceutical Companies. Our main object is to establish bridge between international clients and local expert Clinical Research staff who has Global Experience and Local Expertise. EBM Clinical Research Services Ltd. is already registered to Republic of Turkey Ministry of Health as a Local CRO with 2667269028958 MoH Registration number. Our office is located near the city center in Istanbul, very close to heart of the pharmaceutical industry, and with a beautiful view of the Bosphorus. We believe that the key factor to success in Clinical Research is having a clinical research team that provides clear and exact solutions for clients. We also believe that for better cooperation, partnership and results, local expertise needs global experience.
EBM Clinical Research's clients are pharmaceutical and biotechnology companies and organizations associated with the pharmaceutical industry such as academic research centers, healthcare organizations and government agencies, which benefit from a range of services such as consultancy, data management, clinical trial management and reporting in drug development processes by receiving clinical research services from CROs.
To ensure the quality of EBM Research research, a series of quality assurance steps are implemented, starting from the design of the research to the data collection, analysis and interpretation processes. These steps include measures such as establishing a robust research protocol, selecting appropriate participants, ensuring that data collection and analysis methods comply with standards, testing data accuracy and reliability, and assessing the compliance of results with ethical and scientific standards. In addition, independent audits and expert reviews are conducted during the research process to ensure quality standards and increase the reliability of the research. These methods ensure the quality of EBM Research research and increase the accuracy and reliability of scientific results.